Chemicals/Nitrogens

This is a lecture on the general nature and specific characteristics of various natural and hominin-made nitrogens. It is an offering from the school of chemistry.
The aurora on the right contains an intense violet band above the pink band.
Emissions[edit | edit source]

A nitrogen green emission line occurs in plasmas at 566.934 nm from N VIII.[1]
Nitrogen has an emission line at 658.4 nm.
Nitrogen has two emission lines that occur in plasmas at 455.368 and 455.545 nm from N VII.[1]
There is an "(0,2) vibrational component of the B-x electronic transition of N2(+) at 470.9 nm."[2]
Nitrogen has an emission line that occurs in plasmas at 388.678 nm from N VII.[1]
As seen in its spectrum above, nitrogen has many emission lines in the violet.
Allotropes[edit | edit source]
Atomic nitrogen is an allotrope of nitrogen.
Dinitrogens[edit | edit source]
Given the great reactivity of atomic nitrogen, elemental nitrogen usually occurs as molecular N2, dinitrogen which is a colourless, odourless, and tasteless diamagnetic gas at standard conditions: it melts at −210 °C and boils at −196 °C.[3] Dinitrogen is mostly unreactive at room temperature, but it will nevertheless react with lithium metal and some transition metal complexes due to its bonding, which is unique among the diatomic elements at standard conditions in that it has an N≡N triple bond. Triple bonds have short bond lengths (in this case, 109.76 pm) and high dissociation energies (in this case, 945.41 kJ/mol), and are thus very strong, explaining dinitrogen's chemical inertness.[3]
Cationic and anionic polynitrogens[edit | edit source]
Azide is N−
3, pentazenium (N+
5), and pentazolide (cyclic aromatic N−
5).[4]
Under extremely high pressures (1.1 million atm) and high temperatures (2000 K), as produced in a diamond anvil cell, nitrogen polymerises into the single-bonded cubic gauche crystal structure. This structure is similar to that of diamond, and both have extremely strong covalent bonds, resulting in its nickname "nitrogen diamond".[5]
Atomic nitrogens[edit | edit source]
Atomic nitrogen is prepared by passing an electric discharge through nitrogen gas at 0.1–2 mmHg, which produces atomic nitrogen along with a peach-yellow emission that fades slowly as an afterglow for several minutes even after the discharge terminates.[3]
Subatomics[edit | edit source]

In the nuclide map on the right, orange indicates proton emission (nuclides outside the proton drip line); pink for positron emission (inverse beta decay); black for stable nuclides; blue for electron emission (beta decay); and violet for neutron emission (nuclides outside the neutron drip line). Proton number increases going up the vertical axis and neutron number going to the right on the horizontal axis.
- 10
N - 11
N - 12
N Of the ten other isotopes produced synthetically, ranging from 12N to 23N, 13N has a half-life of ten minutes and the remaining isotopes have half-lives on the order of seconds (16N and 17N) or even milliseconds. No other nitrogen isotopes are possible as they would fall outside the nuclear drip lines, leaking out a proton or neutron.[6] Given the half-life difference, 13N is the most important nitrogen radioisotope, being relatively long-lived enough to use in positron emission tomography (PET), although its half-life is still short and thus it must be produced at the venue of the PET, for example in a cyclotron via proton bombardment of 16O producing 13N and an alpha particle.[7] - 13
N - 14
N Nitrogen has two stable isotopes: 14N and 15N, where the first is much more common, making up 99.634% of natural nitrogen, and the second (which is slightly heavier) makes up the remaining 0.366%, which leads to an atomic weight of around 14.007 u.[8] Both of these stable isotopes are produced in the CNO cycle in stars, but 14N is (1) more common as its neutron capture is the rate-limiting step and (2) 14N is one of the five stable odd–odd nuclides (a nuclide having an odd number of protons and neutrons).[9] - 15
N - 16
N The radioisotope 16N is the dominant radionuclide in the coolant of pressurised water reactors or boiling water reactors during normal operation, and thus it is a sensitive and immediate indicator of leaks from the primary coolant system to the secondary steam cycle, and is the primary means of detection for such leaks. It is produced from 16O (in water) via an (n,p) reaction in which the 16O atom captures a neutron and expels a proton. It has a short half-life of about 7.1 s,[6] but during its decay back to 16O produces high-energy gamma radiation (5 to 7 MeV).[6][10] Because of this, access to the primary coolant piping in a pressurised water reactor must be restricted during reactor power operation.[10] - 17
N - 18
N - 19
N - 20
N - 21
N - 22
N - 23
N - 24
N - 25
N
The relative abundance of 14N and 15N is practically constant in the atmosphere but can vary elsewhere, due to natural isotopic fractionation from biological redox reactions and the evaporation of natural ammonia or nitric acid.[11] Biologically mediated reactions (e.g., assimilation, nitrification, and denitrification) strongly control nitrogen dynamics in the soil, where these reactions typically result in 15N enrichment of the substrate and depletion of the product.[12]
The 15N:14N ratio is commonly used in stable isotope analysis in the fields of geochemistry, hydrology, paleoclimatology and paleoceanography, where it is called δ15
N.[13]
Neutrons[edit | edit source]
"The disintegration of nitrogen by slow neutrons has been studied in photographic emulsions of different sensitivity, which enable an unambiguous distinction to be made between the emission of α-particles and protons. Evidence has been obtained that the disintegration takes place according to the reaction
- 1n + 14
7N
→ 14
5B
+ 4
2He
with a cross-section of about 10−24 cm2."[14]
"The fast neutrons from uranium fission can be moderated by elastic collision with graphite or heavy water in a nuclear reactor until their velocity is reduced to that of thermal energies (average 2200 meters/sec= 0.025 electron volts); such very slow neutrons are called thermal neutrons. Unlike fast neutrons, whose principal reaction with matter is one of scattering, thermal neutrons because of their very low energy and velocity are more likely to be captured than scattered by the atoms they encounter. Most of the reactions of biological elements with thermal neutrons are capture reactions. After an atom captures a neutron, it forms a new compound nucleus with an excess of energy. This new compound nucleus may then:
- emit immediately a capture radiation to form a radioactive daughter which subsequently emits beta or gamma rays at a rate characteristic for the isotope formed, e.g.,
- 14
7N
+ 1n → [15
7N
] → 14
6C
* + p (half life = 6,000 years) → 14
7N
+ β-."[15]
- 14
Protons[edit | edit source]
Nitrogen is element number seven based on the number of protons in its nucleus.
Positrons[edit | edit source]
"Isotopes which undergo this decay and thereby emit positrons include carbon-11, potassium-40, nitrogen-13, oxygen-15, fluorine-18, and iodine-121. As an example, the following equation describes the beta plus decay of carbon-11 to boron-11, emitting a positron and a neutrino:"[16]
Muons[edit | edit source]
"The [cosmic-ray] shower can be observed by: i) sampling the electromagnetic and hadronic components when they reach the ground with an array of particle detectors such as scintillators, ii) detecting the fluorescent light emitted by atmospheric nitrogen excited by the passage of the shower particles, iii) detecting the Cerenkov light emitted by the large number of particles at shower maximum, and iv) detecting muons and neutrinos underground."[17]
Neutrinos[edit | edit source]
"These “atmospheric neutrinos” come from the decay of pions and kaons produced by the collisions of cosmic-ray particles with nitrogen and oxygen in the atmosphere."[18]
Ultraviolets[edit | edit source]
Several emission lines occur in plasmas at 347.872, 348.30, and 348.493 nm from N IV and 252.255, 344.211, and 388.678 nm from N VII.[1]
Molecular nitrogen and nitrogen compounds have been detected in interstellar space by astronomers using the Far Ultraviolet Spectroscopic Explorer.[19]
Visuals[edit | edit source]
"[T]he 5198-, 5201-A lines of nitrogen [occur] in the [Earth's] nightglow."[20]
Violets[edit | edit source]

"Auroras are known to be generated by beams of electrons which are accelerated along Earth's magnetic field lines. The fast-moving electrons collide with atoms in the ionosphere at altitudes of between 100 to 600 km. This interaction with oxygen atoms results in a green or, more rarely, red glow in the night sky, while nitrogen atoms yield blue and purple colours."[21]
The aurora borealis imaged on the right shows blue, violet, and purple colors with the Milky Way in the background.
Blues[edit | edit source]



"When the charged particles from the Sun penetrate Earth's magnetic shield, they are channelled downwards along the magnetic field lines until they strike atoms of gas high in the atmosphere. Like a giant fluorescent neon lamp, the interaction with excited oxygen atoms generates a green or, more rarely, red glow in the night sky, while excited nitrogen atoms yield blue and purple colours."[22]
There is an "(0,2) vibrational component of the B-x electronic transition of N2(+) at 470.9 nm."[2]
The image on the right shows blue aurora borealis that occurred over Iceland.
The second image down on the right shows an extensive blue aurora above the green over Canada.
The image on the left shows an extensive blue aurora.
Cyans[edit | edit source]

Nitrogen appears to have lines near to the cyan.
"M2-9 [in the image at right] is a striking example of a "butterfly" or a bipolar planetary nebula. Another more revealing name might be the "Twin Jet Nebula." If the nebula is sliced across the star, each side of it appears much like a pair of exhausts from jet engines. Indeed, because of the nebula's shape and the measured velocity of the gas, in excess of 200 miles per second, astronomers believe that the description as a super-super-sonic jet exhaust is quite apt. Ground-based studies have shown that the nebula's size increases with time, suggesting that the stellar outburst that formed the lobes occurred just 1,200 years ago."[23]
"The central star in M2-9 is known to be one of a very close pair which orbit one another at perilously close distances. It is even possible that one star is being engulfed by the other. Astronomers suspect the gravity of one star pulls weakly bound gas from the surface of the other and flings it into a thin, dense disk which surrounds both stars and extends well into space."[23]
"The disk can actually be seen in shorter exposure images obtained with the Hubble telescope. It measures approximately 10 times the diameter of Pluto's orbit. Models of the type that are used to design jet engines ("hydrodynamics") show that such a disk can successfully account for the jet-exhaust-like appearance of M2-9. The high-speed wind from one of the stars rams into the surrounding disk, which serves as a nozzle. The wind is deflected in a perpendicular direction and forms the pair of jets that we see in the nebula's image. This is much the same process that takes place in a jet engine: The burning and expanding gases are deflected by the engine walls through a nozzle to form long, collimated jets of hot air at high speeds."[23]
"M2-9 is 2,100 light-years away in the constellation Ophiucus. The observation was taken Aug. 2, 1997 by the Hubble telescope's Wide Field and Planetary Camera 2. In this image, neutral oxygen is shown in red, once-ionized nitrogen in green, and twice-ionized oxygen in blue."[23]
Yellows[edit | edit source]
Nitrogen has a yellow forbidden line, specifically N II at 575.5 nm, that may be used to indicate nitrogen abundances and contribute to nitrogen/oxygen (N/O) abundance gradients. Surveys of H II regions in spiral galaxies have suggested that N/O abundance ratios increase from outer-arm nebulae to inner-arm nebulae.[24] "Electron temperatures are generally derived from the ratio of auroral to nebular lines in [O III] or [N II]."[25] "[B]ecause of the proximity of strong night-sky lines at λ4358 and λλ5770, 5791, the auroral lines of [O III] λ4363 and [N II] λ5755 are often contaminated."[25]
Oranges[edit | edit source]
Nitrogen has a weak line in the orange.
Nucleosynthesis[edit | edit source]
The carbon-burning process is a set of nuclear reactions that may require high temperatures (> 5×108 K or 50 keV) and densities (> 3×109 kg/m3).[26]
CNO-I[edit | edit source]
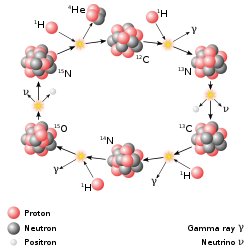
The principal reactions are:[27]"
12
6C
+ 1
1H
→ 13
7N
+ γ + 1.95 MeV
13
7N
→ 13
6C
+ e+ + ν
e + 1.20 MeV (half-life of 9.965 minutes[28]), or 12C(p,γ)13N.
13
6C
+ 1
1H
→ 14
7N
+ γ + 7.54 MeV
14
7N
+ 1
1H
→ 15
8O
+ γ + 7.35 MeV
15
8O
→ 15
7N
+ e+
+ ν
e + 1.73&bsp;MeV (half-life of 122.24 seconds[28])
15
7N
+ 1
1H
→ 12
6C
+ 4
2He
+ 4.96 MeV:[29]
CNO-II[edit | edit source]
15
7N
+ 1
1H
→ 16
8O
+ γ + 12.13 MeV
16
8O
+ 1
1H
→ 17
9F
+ γ + 0.60 MeV
17
9F
→ 17
8O
+ e+
+ ν
e + 2.76 MeV (half-life of 64.49 seconds)
17
8O
+ 1
1H
→ 14
7N
+ 4
2He
+ 1.19 MeV
14
7N
+ 1
1H
→ 15
8O
+ γ + 7.35 MeV
15
8O
→ 15
7N
+ e+
+ ν
e + 2.75 MeV (half-life of 122.24 seconds)
CNO-III[edit | edit source]
17
8O
+ 1
1H
→ 18
9F
+ γ + 5.61 MeV
18
9F
→ 18
8O
+ e+
+ ν
e + 1.656 MeV (half-life of 109.771 minutes)
18
8O
+ 1
1H
→ 15
7N
+ 4
2He
+ 3.98 MeV
15
7N
+ 1
1H
→ 16
8O
+ γ + 12.13 MeV
16
8O
+ 1
1H
→ 17
9F
+ γ + 0.60 MeV
17
9F
→ 17
8O
+ e+
+ ν
e + 2.76 MeV (half-life of 64.49 seconds)
CNO-IV[edit | edit source]

These reactions may occur in massive stars.
19
9F
+ 1
1H
→ 16
8O
+ 4
2He
+ 8.114 MeV
16
8O
+ 1
1H
→ 17
9F
+ γ + 0.60 MeV
17
9F
→ 17
8O
+ e+
+ ν
e + 2.76 MeV (half-life of 64.49 seconds)
17
8O
+ 1
1H
→ 18
9F
+ γ + 5.61 MeV
18
9F
→ 18
8O
+ e+
+ ν
e + 1.656 MeV (half-life of 109.771 minutes)
18
8O
+ 1
1H
→ 19
9F
+ γ + 7.994 MeV
HCNO-I[edit | edit source]
These are the hot CNO cycles reactions with conditions of higher temperature as have been found in novae and X-ray bursts.
When the rate of proton captures exceeds the rate of beta-decay, the burning conforms to the proton drip line. A radioactive species captures a proton before it can beta decay.
12
6C
+ 1
1H
→ 13
7N
+ γ + 1.95 MeV
13
7N
+ 1
1H
→ 14
8O
+ γ + 4.63 MeV
14
8O
→ 14
7N
+ e+
+ ν
e + 5.14 MeV (half-life of 70.641 seconds)
14
7N
+ 1
1H
→ 15
8O
+ γ + 7.35 MeV
15
8O
→ 15
7N
+ e+
+ ν
e + 2.75 MeV (half-life of 122.24 seconds)
15
7N
+ 1
1H
→ 12
6C
+ 4
2He
+ 4.96 MeV
HCNO-II[edit | edit source]
15
7N
+ 1
1H
→ 16
8O
+ γ + 12.13 MeV
16
8O
+ 1
1H
→ 17
9F
+ γ + 0.60 MeV
17
9F
+ 1
1H
→ 18
10Ne
+ γ + 3.92 MeV
18
10Ne
→ 18
9F
+ e+
+ ν
e + 4.44 MeV (half-life of 1.672 seconds)
18
9F
+ 1
1H
→ 15
8O
+ 4
2He
+ 2.88 MeV
15
8O
→ 15
7N
+ e+
+ ν
e + 2.75 MeV (half-life of 122.24 seconds)
HCNO-III[edit | edit source]
18
9F
+ 1
1H
→ 19
10Ne
+ γ + 6.41 MeV
19
10Ne
→ 19
9F
+ e+
+ ν
e + 3.32 MeV (half-life of 17.22 seconds)
19
9F
+ 1
1H
→ 16
8O
+ 4
2He
+ 8.11 MeV
16
8O
+ 1
1H
→ 17
9F
+ γ + 0.60 MeV
17
9F
+ 1
1H
→ 18
10Ne
+ γ + 3.92 MeV
18
10Ne
→ 18
9F
+ e+
+ ν
e + 4.44 MeV (half-life of 1.672 seconds)
Plasmas[edit | edit source]
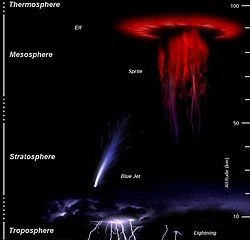
Blue jets differ from sprites in that they project from the top of the cumulonimbus above a thunderstorm, typically in a narrow cone, to the lowest levels of the ionosphere 40 to 50 km (25 to 30 miles) above the earth, whereas red sprites tend to be associated with significant lightning strikes, blue jets do not appear to be directly triggered by lightning (they do, however, appear to relate to strong hail activity in thunderstorms).[30]
They are also brighter than sprites and, as implied by their name, are blue in color. The color is believed to be due to a set of blue and near-ultraviolet emission lines from neutral and ionized molecular nitrogen."[31]
Gases[edit | edit source]

"Molecular nitrogen (N2) [is] a colorless, odorless gas at room temperature."[32]
Cyanides[edit | edit source]
"The CN radical through emission in its Violet system bands has a long-established presence in comets."[33]
Liquids[edit | edit source]

On the right liquid diatomic nitrogen (N2) is being poured.
Liquid nitrogen, a colourless fluid resembling water in appearance, but with 80.8% of the density (the density of liquid nitrogen at its boiling point is 0.808 g/mL), is a common cryogen.[34]
Solids[edit | edit source]

At atmospheric pressure, molecular nitrogen condenses (liquefies) at 77 K (−195.79 °C) and freezes at 63 K (−210.01 °C)[35] into the beta hexagonal close-packed crystal allotropic form. Below 35.4 K (−237.6 °C) nitrogen assumes the cubic crystal allotropic form (called the alpha phase).[36]
Solid nitrogen has many crystalline modifications, forming a significant dynamic surface coverage on Pluto[37] and outer moons of the Solar System such as Triton.[38] Even at the low temperatures of solid nitrogen it is fairly volatile and can sublime]] to form an atmosphere, or condense back into nitrogen frost. It is very weak and flows in the form of glaciers and on Triton geysers of nitrogen gas come from the polar ice cap region.[39]
"In this highest-resolution image [on the right] from NASA's New Horizons spacecraft, great blocks of Pluto's water-ice crust appear jammed together in the informally named al-Idrisi mountains. Some mountain sides appear coated in dark material, while other sides are bright. Several sheer faces appear to show crustal layering, perhaps related to the layers seen in some of Pluto's crater walls. Other materials appear crushed between the mountains, as if these great blocks of water ice, some standing as much as 1.5 miles high, were jostled back and forth. The mountains end abruptly at the shoreline of the informally named Sputnik Planum, where the soft, nitrogen-rich ices of the plain form a nearly level surface, broken only by the fine trace work of striking, cellular boundaries and the textured surface of the plain's ices (which is possibly related to sunlight-driven ice sublimation). This view is about 50 miles wide. The top of the image is to Pluto's northwest."[40]
"These images were made with the telescopic Long Range Reconnaissance Imager (LORRI) aboard New Horizons, in a timespan of about a minute centered on 11:36 UT on July 14 -- just about 15 minutes before New Horizons' closest approach to Pluto -- from a range of just 10,000 miles (17,000 kilometers). They were obtained with an unusual observing mode; instead of working in the usual "point and shoot," LORRI snapped pictures every three seconds while the Ralph/Multispectral Visual Imaging Camera (MVIC) aboard New Horizons was scanning the surface. This mode requires unusually short exposures to avoid blurring the images."[40]
Radiocarbon dating[edit | edit source]
"Initially, the 14
C atoms get oxidized to 14
CO, primarily (Pandow et al. 1960; MacKay et al. (1963):"[41]
- C(g) + O2 ⟶ CO + O: ∆H = –138.96kcal
"In the atmosphere, initially 90–100% of the 14
C produced is oxidized to 14
CO (Pandow et al. 1960)."[41]
"In the atmosphere, the main removal process for 14
CO is oxidation by OH radicals (cf. Jockel et al. 1999). Because of the low abundance of CO in the atmosphere, and the formation of 14
CO initially, the 14
C/12
C ratio in the atmospheric CO is much higher than in the atmospheric CO
2; by about two orders of magnitude in the lower stratosphere (Brenninkmeijer et al. 1995)."[41]
Titan[edit | edit source]

"The atmosphere of Titan is largely composed of nitrogen; minor components lead to the formation of methane and ethane clouds and nitrogen-rich organic smog."[42]
Nebulas[edit | edit source]


At right is an image gaseous objects ("cometary knots") discovered in the thousands. These knots are imaged with the Hubble Space Telescope while exploring the Helix nebula, the closest planetary nebula to Earth at 450 light-years away in the constellation Aquarius. Although ground-based telescopes have revealed such objects, astronomers have never seen so many of them. The most visible knots all lie along the inner edge of the doomed star's ring, trillions of miles away from the star's nucleus. Although these gaseous knots appear small, they're actually huge. Each gaseous head is at least twice the size of our solar system; each tail stretches for 100 billion miles, about 1,000 times the distance between the Earth and the Sun. The image was taken in August 1994 with Hubble's Wide Field Planetary Camera 2. The red light depicts nitrogen emission ([NII] 658.4 nm).
The left image is a color picture, taken with the Wide Field Planetary Camera-2. It is a composite of three images taken at different wavelengths. (red, hydrogen-alpha; blue, neutral oxygen, 630.0 nm; green, ionized nitrogen, 658.4 nm). This NASA Hubble Space Telescope image shows one of the most complex planetary nebulae ever seen, NGC 6543, nicknamed the "Cat's Eye Nebula." The image was taken on September 18, 1994. NGC 6543 is 3,000 light-years away in the northern constellation Draco. The term planetary nebula is a misnomer; dying stars create these cocoons when they lose outer layers of gas.
Milky Way[edit | edit source]
"The nitrogen abundance appears to increase with decreasing galactocentric distance. ... A least-squares solution weighting the points equally gives a magnitude for the gradient d(log N/H)/dr = -0.10 ± 0.03 kpc-1."[25] "The ratio N/O clearly increases with decreasing R. A least-squares fit to the data ... gives d(log N/O)/dr = -0.06 ± 0.02 kpc-1."[25]
Technology[edit | edit source]

"Plasma cleaning involves the removal of impurities and contaminants from surfaces through the use of an energetic plasma created from gaseous species. Gases such as argon and oxygen, as well as mixtures such as air and hydrogen/nitrogen are used. The plasma is created by using high frequency voltages (typically kHz to >MHz) to ionise the low pressure gas (typically around 1/1000 atmospheric pressure), although atmospheric pressure plasmas are now also common."[43]
Hypotheses[edit | edit source]
- To form a nitrogen plasma, diatomic molecular nitrogen gas must be dissociated into monatomic nitrogen gas, then one or more electrons must be either added or taken away.
Resources[edit | edit source]
See also[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 K. J. McCarthy; A. Baciero; B. Zurro; TJ-II Team (June 12, 2000). Impurity Behaviour Studies in the TJ-II Stellarator, In: 27th EPS Conference on Contr. Fusion and Plasma Phys.. 24B. Budapest: ECA. pp. 1244-7. http://crpppc42.epfl.ch/Buda/pdf/p3_116.pdf. Retrieved 2013-01-20.
- ↑ 2.0 2.1 C. B. Collins; J. M. Carroll; K. N. Taylor; F. W. Lee (October 1, 1978). "A regenerative power amplifier operating on the blue-green line of the nitrogen ion laser". Applied Physics Letters 33 (7): 624-6. doi:10.1063/1.90484.
- ↑ 3.0 3.1 3.2 Greenwood and Earnshaw, pp. 412–16
- ↑ Lewars, Errol G. (2008). Modeling Marvels: Computational Anticipation of Novel molecules. Springer Science+Business Media. pp. 141–63. doi:10.1007/978-1-4020-6973. ISBN 978-1-4020-6972-7.
- ↑ "Polymeric nitrogen synthesized". physorg.com. 5 August 2004. Retrieved 2009-06-22.
- ↑ 6.0 6.1 6.2 Audi, G.; Wapstra, A. H.; Thibault, C.; Blachot, J.; Bersillon, O. (2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A 729 (1): 3–128. doi:10.1016/j.nuclphysa.2003.11.001. https://web.archive.org/web/20080923135135/http://www.nndc.bnl.gov/amdc/nubase/Nubase2003.pdf.
- ↑ Carlson, Neil (January 22, 2012). Physiology of Behavior. Methods and Strategies of Research. 11th edition. Pearson. p. 151. ISBN 978-0-205-23939-9.
- ↑ Greenwood and Earnshaw, pp. 411–12
- ↑ Bethe, H. A. (1939). "Energy Production in Stars". Physical Review 55 (5): 434–56. doi:10.1103/PhysRev.55.434.
- ↑ 10.0 10.1 Neeb, Karl Heinz (1997). The Radiochemistry of Nuclear Power Plants with Light Water Reactors. Berlin-New York: Walter de Gruyter. p. 227. ISBN 978-3-11-013242-7. https://books.google.com/books?id=SJOE00whg44C&pg=PA227.
- ↑ CIAAW (2003). "Atomic Weight of Nitrogen". CIAAW. Retrieved 13 October 2016.
- ↑ Flanagan, Lawrence B.; Ehleringer, James R.; Pataki, Diane E. (15 December 2004). Stable Isotopes and Biosphere – Atmosphere Interactions: Processes and Biological Controls. pp. 74–75. ISBN 978-0-08-052528-0. https://books.google.com/books?id=U9y3whFC2DIC&pg=PA74.
- ↑ Katzenberg, M. A. (2008). "Chapter 13: Stable Isotope Analysis: A Tool for Studying Past Diet, Demography, and Life History". Biological Anthropology of the Human Skeleton (2nd ed.). ISBN 978-0-471-79372-4.
- ↑ W. E. Burcham; M. Goldhaber (December 1936). "The disintegration of nitrogen by slow neutrons". Mathematical Proceedings of the Cambridge Philosophical Society 32 (4): 632-636. https://www.cambridge.org/core/journals/mathematical-proceedings-of-the-cambridge-philosophical-society/article/the-disintegration-of-nitrogen-by-slow-neutrons/4FE09B2AAEC596C3B284C7FC464663D4. Retrieved 2017-11-26.
- ↑ Alan D. Conger; Norman H. Giles Jr. (July 1950). "The Cytogenetic Effect of Slow Neutrons". Genetics 35 (4): 397–419. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1209493/pdf/397.pdf. Retrieved 2017-11-26.
- ↑ "Positron emission, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. January 23, 2014. Retrieved 2014-01-30.
- ↑ Francis Halzen; Dan Hooper (June 12, 2002). "High-energy neutrino astronomy: the cosmic ray connection". Reports on Progress in Physics 65 (7): 1025-107. doi:10.1088/0034-4885/65/7/201. http://iopscience.iop.org/0034-4885/65/7/201. Retrieved 2014-02-08.
- ↑ Francis Halzen; Spencer R. Klein (August 2010). "IceCube: An instrument for neutrino astronomy". Review of Scientific Instruments 81 (8): 081101 - 081101-24. doi:10.1063/1.3480478. http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5574379. Retrieved 2014-02-08.
- ↑ Meyer, Daved M.; Cardelli, Jason A.; Sofia, Ulysses J. (1997). "Abundance of Interstellar Nitrogen". The Astrophysical Journal 490: L103–6. doi:10.1086/311023.
- ↑ D. R. Bates (October 1978). "Forbidden oxygen and nitrogen lines in the nightglow". Planetary and Space Science 26 (10): 897-912. doi:10.1016/0032-0633(78)90073-9. http://www.sciencedirect.com/science/article/pii/0032063378900739. Retrieved 2013-01-16.
- ↑ Andrew Wright (9 April 2015). "Heart of the Black Auroras Revealed by Cluster". European Space Agency. Retrieved 2015-04-12.
- ↑ European Space Agency (9 April 2015). "Aurora over Icelandic Lake". ESA. Retrieved 2015-04-12.
- ↑ 23.0 23.1 23.2 23.3 Bruce Balick (December 17, 1997). "Supersonic Exhaust from Nebula M2-9". Baltimore, Maryland USA: Hubble Site. Retrieved 2014-02-26.
- ↑ C. L. Searle (1971). The Astrophysical Journal 168: 327.
- ↑ 25.0 25.1 25.2 25.3 S. A. Hawley (September 1, 1978). "The chemical composition of galactic and extragalactic H II regions". The Astrophysical Journal 224 (9): 417-36. doi:10.1086/156389.
- ↑ Sean G. Ryan; Andrew J. Norton (2010). Stellar Evolution and Nucleosynthesis. Cambridge University Press. p. 135. ISBN 978-0-521-13320-3. http://books.google.com/?id=PE4yGiU-JyEC#v=onepage&f=false.
- ↑ W. H. Camiel; C. Doom de Loore (1992). "Structure and evolution of single and binary stars". In Camiel W. H. de Loore. Volume 179 of Astrophysics and space science library. Springer. pp. 95–97. ISBN 978-0-7923-1768-5. http://books.google.com/?id=LJgNIi0vkeYC#v=snippet&f=false.
- ↑ 28.0 28.1 Principles and Perspectives in Cosmochemistry, Springer, 2010, ISBN 9783642103681, page 233
- ↑ Krane, K. S. (1988). Introductory Nuclear Physics. John Wiley & Sons. p. 537. ISBN 0-471-80553-X.
- ↑ "Fractal Models of Blue Jets, Blue Starters Show Similarity, Differences to Red Sprites".
- ↑ "Upper atmospheric lightning". San Francisco, California: Wikimedia Foundation, Inc. April 29, 2013. Retrieved 2013-05-28.
- ↑ "nitrogen, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. September 22, 2013. Retrieved 2013-10-05.
- ↑ S.R. Federman; David L. Lambert (May 2002). "The need for accurate oscillator strengths and cross sections in studies of diffuse interstellar clouds and cometary atmospheres". Journal of Electron Spectroscopy and Related Phenomena 123 (2-3): 161-71. http://www.sciencedirect.com/science/article/pii/S0368204802000178. Retrieved 2013-01-20.
- ↑ Iancu, C. V.; Wright, E. R.; Heymann, J. B.; Jensen, G. J. (2006). "A comparison of liquid nitrogen and liquid helium as cryogens for electron cryotomography". Journal of Structural Biology 153 (3): 231–40. doi:10.1016/j.jsb.2005.12.004. PMID 16427786.
- ↑ Gray, Theodore (2009). The Elements: A Visual Exploration of Every Known Atom in the Universe. New York: Black Dog & Leventhal Publishers. ISBN 978-1-57912-814-2.
- ↑ Schuch, A. F.; Mills, R. L. (1970). "Crystal Structures of the Three Modifications of Nitrogen 14 and Nitrogen 15 at High Pressure". The Journal of Chemical Physics 52 (12): 6000–08. doi:10.1063/1.1672899.
- ↑ "Flowing nitrogen ice glaciers seen on surface of Pluto after New Horizons flyby". 25 July 2015. Retrieved 6 October 2015.
- ↑ McKinnon, William B.; Kirk, Randolph L. (2014). TilmanSpohn. ed. Triton, In: Encyclopedia of the Solar System (3rd ed.). Amsterdam; Boston: Elsevier. pp. 861–82. ISBN 978-0-12-416034-7. https://books.google.com/books?id=0bEMAwAAQBAJ&pg=PA861.
- ↑ "Neptune: Moons: Triton". NASA. Retrieved September 21, 2007.
- ↑ 40.0 40.1 Sue Lavoie (14 July 2015). PIA20198: The Mountainous Shoreline of Sputnik Planum. Pasadena, California USA: NASA/JPL. https://photojournal.jpl.nasa.gov/catalog/PIA20198. Retrieved 11 January 2019.
- ↑ 41.0 41.1 41.2 D Lal; A J T Jull (2001). "In-situ cosmogenic 14
C: Production and examples of its unique applications in studies of terrestrial and extraterrestrial processes". Radiocarbon 43 (28): 731-742. https://journals.uair.arizona.edu/index.php/radiocarbon/article/download/3905/3330. Retrieved 2017-12-06. - ↑ "Titan (moon)". San Francisco, California: Wikimedia Foundation, Inc. June 30, 2012. Retrieved 2012-07-01.
- ↑ "Plasma cleaning". San Francisco, California: Wikimedia Foundation, Inc. April 13, 2013. Retrieved 2013-05-28.
