Malaria
Malaria is a vector-borne infectious disease that is widespread in tropical and subtropical regions, including parts of the Americas, Asia, and Africa. Each year, it causes disease in approximately 650 million people and kills between one and three million, most of them young children in Sub-Saharan Africa. Malaria is commonly-associated with poverty, but is also a cause of poverty and a major hindrance to economic development.
Malaria is one of the most common infectious diseases and an enormous public-health problem. The disease is caused by protozoan parasites of the genus Plasmodium. The most serious forms of the disease are caused by Plasmodium falciparum and Plasmodium vivax, but other related species (Plasmodium ovale, Plasmodium malariae, and sometimes Plasmodium knowlesi) can also infect humans. This group of human-pathogenic Plasmodium species is usually referred to as malaria parasites.
Malaria parasites are transmitted by female Anopheles mosquitoes. The parasites multiply within red blood cells, causing symptoms that include symptoms of anemia (light headedness, shortness of breath, tachycardia etc.), as well as other general symptoms such as fever, chills, nausea, flu-like illness, and in severe cases, coma and death. Malaria transmission can be reduced by preventing mosquito bites with mosquito nets and insect repellents, or by mosquito control by spraying insecticides inside houses and draining standing water where mosquitoes lay their eggs.
No vaccine is currently available for malaria; preventative drugs must be taken continuously to reduce the risk of infection. These prophylactic drug treatments are often too expensive for most people living in endemic areas. Most adults from endemic areas have a degree of long-term recurrent infection and also of partial resistance; the resistance reduces with time and such adults may become susceptible to severe malaria if they have spent a significant amount of time in non-endemic areas. They are strongly recommended to take full precautions if they return to an endemic area. Malaria infections are treated through the use of antimalarial drugs, such as quinine or artemisinin derivatives, although drug resistance is increasingly common.
Causes[edit | edit source]
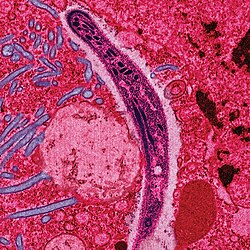
Malaria parasites[edit | edit source]
Malaria is caused by protozoan parasites of the genus Plasmodium (phylum Apicomplexa). In humans malaria is caused by P. falciparum, P. malariae, P. ovale, and P. vivax. However, P. falciparum is the most important cause of disease and responsible for about 80% of infections and 90% of deaths.[1] Parasitic Plasmodium species also infect birds, reptiles, monkeys, chimpanzees and rodents.[2] There have been documented human infections with several simian species of malaria, namely P. knowlesi, P. inui, P. cynomolgi[3], P. simiovale, P. brazilianum, P. schwetzi and P. simium; however these are mostly of limited public health importance. Although avian malaria can kill chickens and turkeys, this disease does not cause serious economic losses to poultry farmers.[4] However, since being accidentally introduced by humans it has decimated the endemic birds of Hawaii, which evolved in its absence and lack any resistance to it.[5]
Pathogenesis[edit | edit source]
Malaria in humans develops via two phases: an exoerythrocytic (hepatic) and an erythrocytic phase. When an infected mosquito pierces a person's skin to take a blood meal, sporozoites in the mosquito's saliva enter the bloodstream and migrate to the liver. Within 30 minutes of being introduced into the human host, they infect hepatocytes, multiplying asexually and asymptomatically for a period of 6–15 days. During this so-called dormant time in the liver, the sporozoites are often referred to as hypnozoites.[6] Once in the liver, these organisms differentiate to yield thousands of merozoites which, following rupture of their host cells, escape into the blood and infect red blood cells, thus beginning the erythrocytic stage of the life cycle.[7] The parasite escapes from the liver undetected by wrapping itself in the cell membrane of the infected host liver cell.[8]
Within the red blood cells the parasites multiply further, again asexually, periodically breaking out of their hosts to invade fresh red blood cells. Several such amplification cycles occur. Thus, classical descriptions of waves of fever arise from simultaneous waves of merozoites escaping and infecting red blood cells.
Some P. vivax and P. ovale sporozoites do not immediately develop into exoerythrocytic-phase merozoites, but instead produce hypnozoites that remain dormant for periods ranging from several months (6–12 months is typical) to as long as three years. After a period of dormancy, they reactivate and produce merozoites. Hypnozoites are responsible for long incubation and late relapses in these two species of malaria.[9] This is conventional view. However, it is now becoming recognized that relapse-like recurrent P. vivax malaria might frequently have a hypnozoite-independent origin(s).[10] The parasite is relatively protected from attack by the body's immune system because for most of its human life cycle it resides within the liver and blood cells and is relatively invisible to immune surveillance. However, circulating infected blood cells are destroyed in the spleen. To avoid this fate, the P. falciparum parasite displays adhesive proteins on the surface of the infected blood cells, causing the blood cells to stick to the walls of small blood vessels, thereby sequestering the parasite from passage through the general circulation and the spleen.[11] This "stickiness" is the main factor giving rise to hemorrhagic complications of malaria. High endothelial venules (the smallest branches of the circulatory system) can be blocked by the attachment of masses of these infected red blood cells. The blockage of these vessels causes symptoms such as in placental and cerebral malaria. In cerebral malaria the sequestrated red blood cells can breach the blood brain barrier possibly leading to coma.[12]
Although the red blood cell surface adhesive proteins (called PfEMP1, for Plasmodium falciparum erythrocyte membrane protein 1) are exposed to the immune system they do not serve as good immune targets because of their extreme diversity; there are at least 60 variations of the protein within a single parasite and perhaps limitless versions within parasite populations.[11] Like a thief changing disguises or a spy with multiple passports, the parasite switches between a broad repertoire of PfEMP1 surface proteins, thus staying one step ahead of the pursuing immune system.
Some merozoites turn into male and female gametocytes. If a mosquito pierces the skin of an infected person, it potentially picks up gametocytes within the blood. Fertilization and sexual recombination of the parasite occurs in the mosquito's gut, thereby defining the mosquito as the definitive host of the disease. New sporozoites develop and travel to the mosquito's salivary gland, completing the cycle. Pregnant women are especially attractive to the mosquitoes,[13] and malaria in pregnant women is an important cause of stillbirths, infant mortality and low birth weight.[14]
External links[edit | edit source]
References[edit | edit source]
- ↑ Mendis K, Sina B, Marchesini P, Carter R (2001). "The neglected burden of Plasmodium vivax malaria.". Am J Trop Med Hyg 64 (1-2 Suppl): 97-106. PMID 11425182. http://www.ajtmh.org/cgi/reprint/64/1_suppl/97.pdf.
- ↑ Escalante A, Ayala F (1994). "Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences.". Proc Natl Acad Sci U S A 91 (24): 11373-7. PMID 7972067. http://www.pnas.org/cgi/reprint/91/24/11373.
- ↑ Garnham, PCC (1966). Malaria parasites and other haemosporidia. Oxford: Blackwell Scientific Publications.
- ↑ Investing in Animal Health Research to Alleviate Poverty. International Livestock Research Institute. Permin A. and Madsen M. (2001) Appendix 2: review on disease occurrence and impact (smallholder poultry). Accessed 29 Oct 2006
- ↑ Atkinson CT, Woods KL, Dusek RJ, Sileo LS, Iko WM (1995). "Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected iiwi (Vestiaria coccinea)". Parasitology 111 Suppl: S59-69. PMID 8632925.
- ↑ "Malaria: Origin of the Term “Hypnozoite”". Journal of the History of Biology 44 (4): 781–786. 2011. doi:10.1007/s10739-010-9239-3. PMID 20665090.
- ↑ Bledsoe, G. H. (December 2005) "Malaria primer for clinicians in the United States" Southern Medical Journal 98(12): pp. 1197-204, (PMID: 16440920);
- ↑ Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT (2006). "Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids". Science 313: 1287-1490. PMID 16888102.
- ↑ Cogswell F (1992). "The hypnozoite and relapse in primate malaria.". Clin Microbiol Rev 5 (1): 26-35. PMID 1735093. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=358221&blobtype=pdf.
- ↑ "Biological Concepts in Recurrent Plasmodium vivax Malaria". Parasitology. 2018. doi:10.1017/S003118201800032X. PMID 29564998.
- ↑ 11.0 11.1 Chen Q, Schlichtherle M, Wahlgren M (2000). "Molecular aspects of severe malaria.". Clin Microbiol Rev 13 (3): 439-50. PMID 10885986. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=10885986.
- ↑ Adams S, Brown H, Turner G (2002). "Breaking down the blood-brain barrier: signaling a path to cerebral malaria?". Trends Parasitol 18 (8): 360-6. PMID 12377286.
- ↑ Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G (2000). "Effect of pregnancy on exposure to malaria mosquitoes.". Lancet 355 (9219): 1972. PMID 10859048.
- ↑ van Geertruyden J, Thomas F, Erhart A, D'Alessandro U (2004). "The contribution of malaria in pregnancy to perinatal mortality.". Am J Trop Med Hyg 71 (2 Suppl): 35-40. PMID 15331817. http://www.ajtmh.org/cgi/content/full/71/2_suppl/35.
See more on:Malaria